Hydrogen Safety
Researches on laminar flame are essential for the development of combustion chemistry, ignition problem, flame stability ...
Hydrogen is viewed as a promising energy carrier worldwide in the perspective of the carbon-neutrality goal. The reactive nature of hydrogen involving wide flammability range, low ignition energy and fast flame speed poses the risk of ignition and explosion. The ongoing research aims to secure the safe deployment of hydrogen technologies.
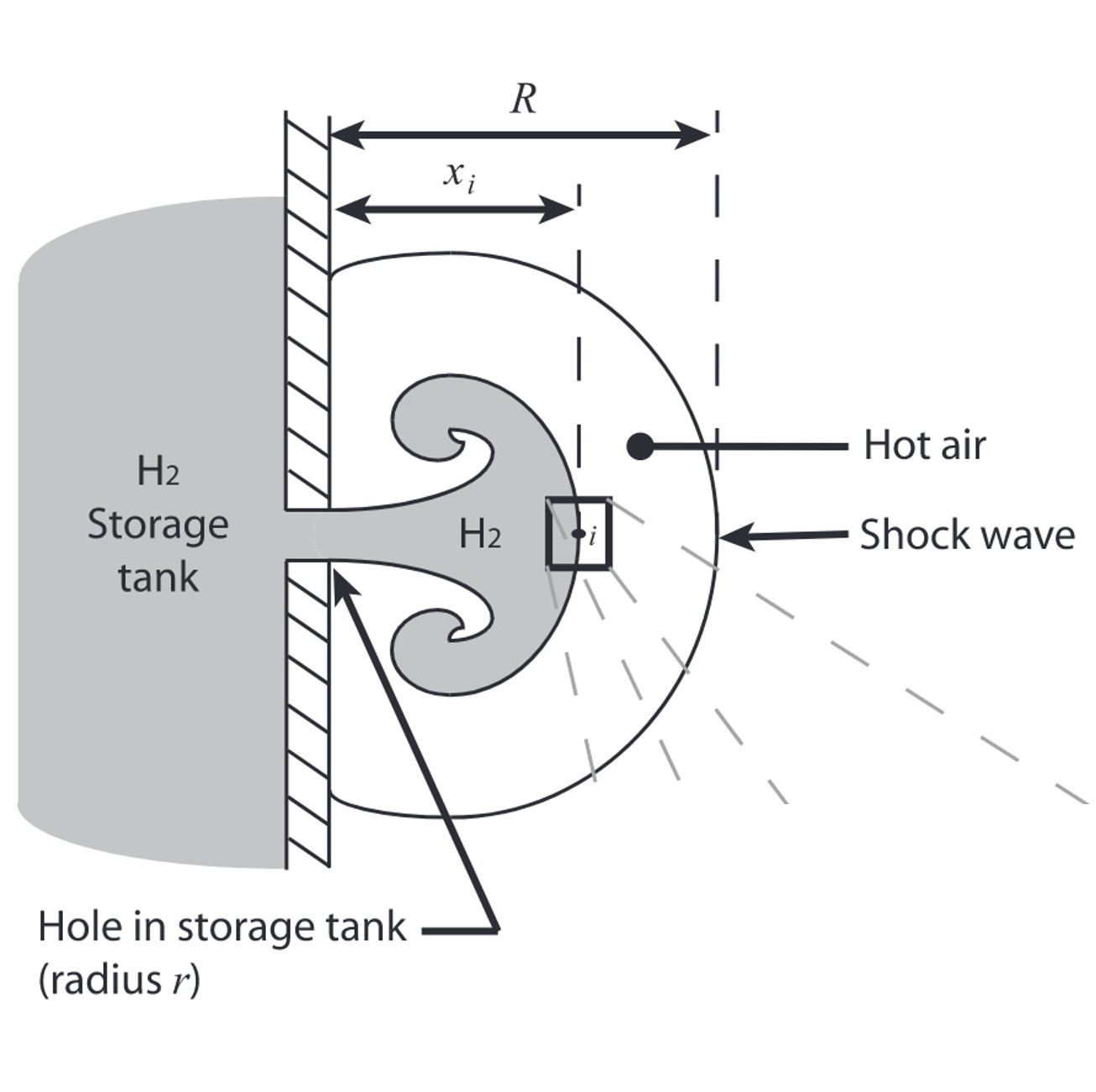
Spontaneous ignition of hydrogen jet
Due to the low volumetric energy density, compressing hydrogen to elevated pressure, i.e., 350 to 700 atm, constitutes a competitive approach for the storage and delivery. However, the spontaneous ignition of hydrogen accidentally released from pressurized equipment poses safety risk to the utilization of hydrogen. A real gas based low-order model is developed by extending Maxwell and Radulescu's work (Combustion and Flame 158, 2011). The problem is simplified as the ignition of a diffusion layer at the jet head.
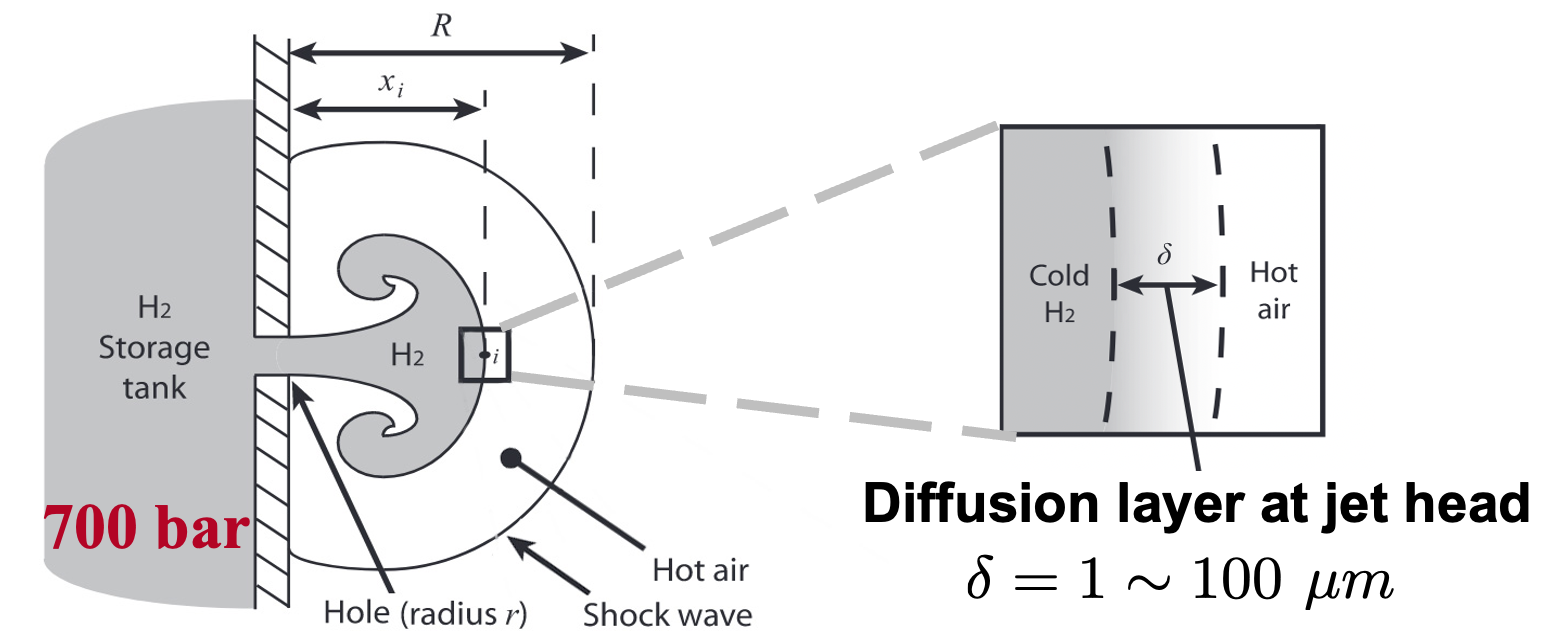
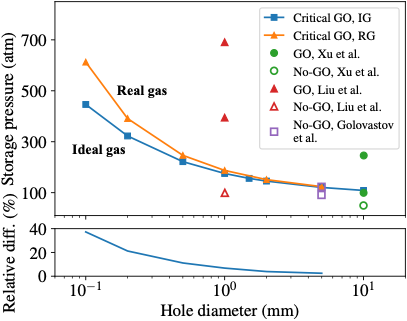
The critical pressure of spontaneous ignition was determined. The results demonstrate satisfactory agreement with experimental data. In unconfined releases, real gas effects lead to higher critical pressures. The relative difference was 10% for a 0.6 mm hole and increased drastically as the hole size was decreased.
Laminar flame speed
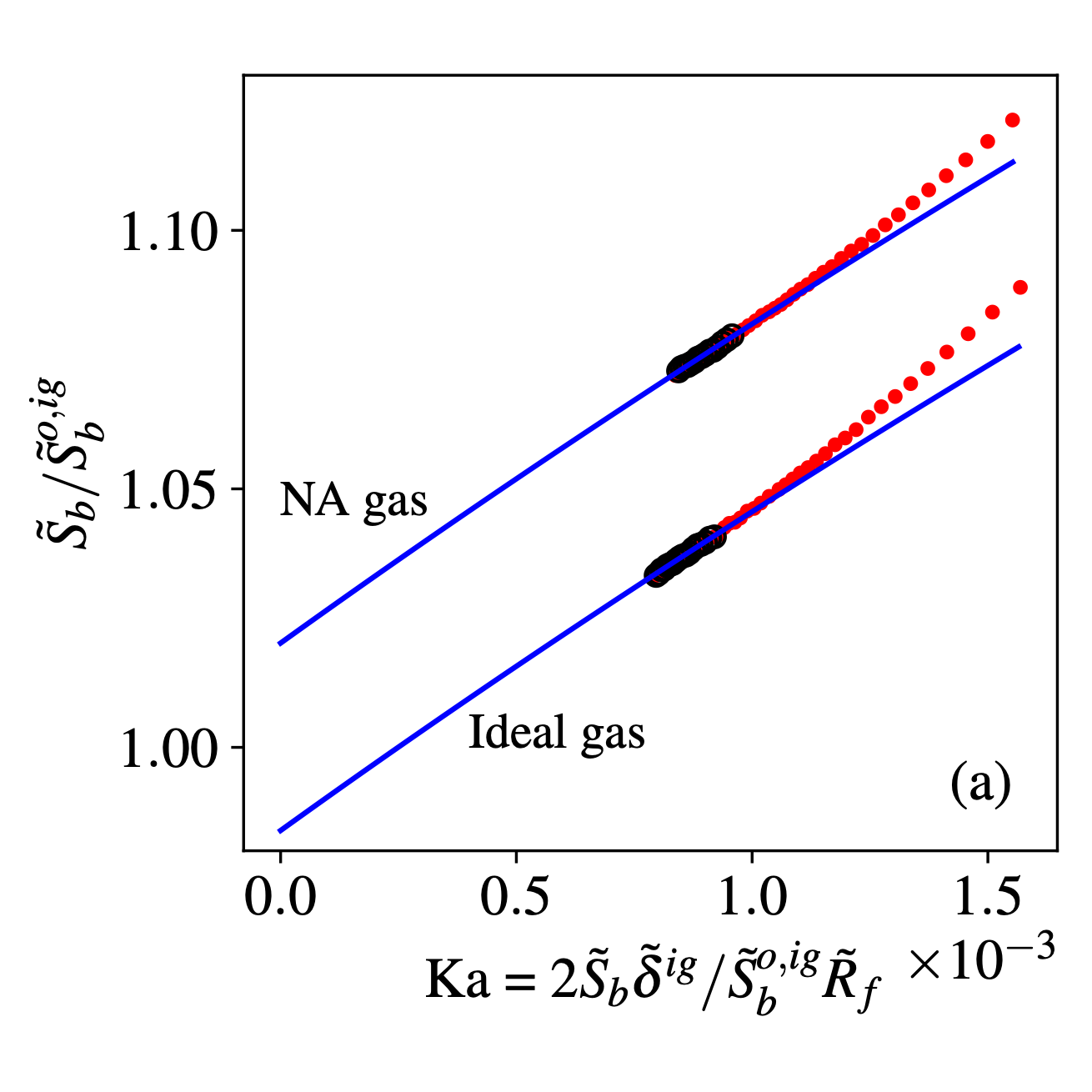
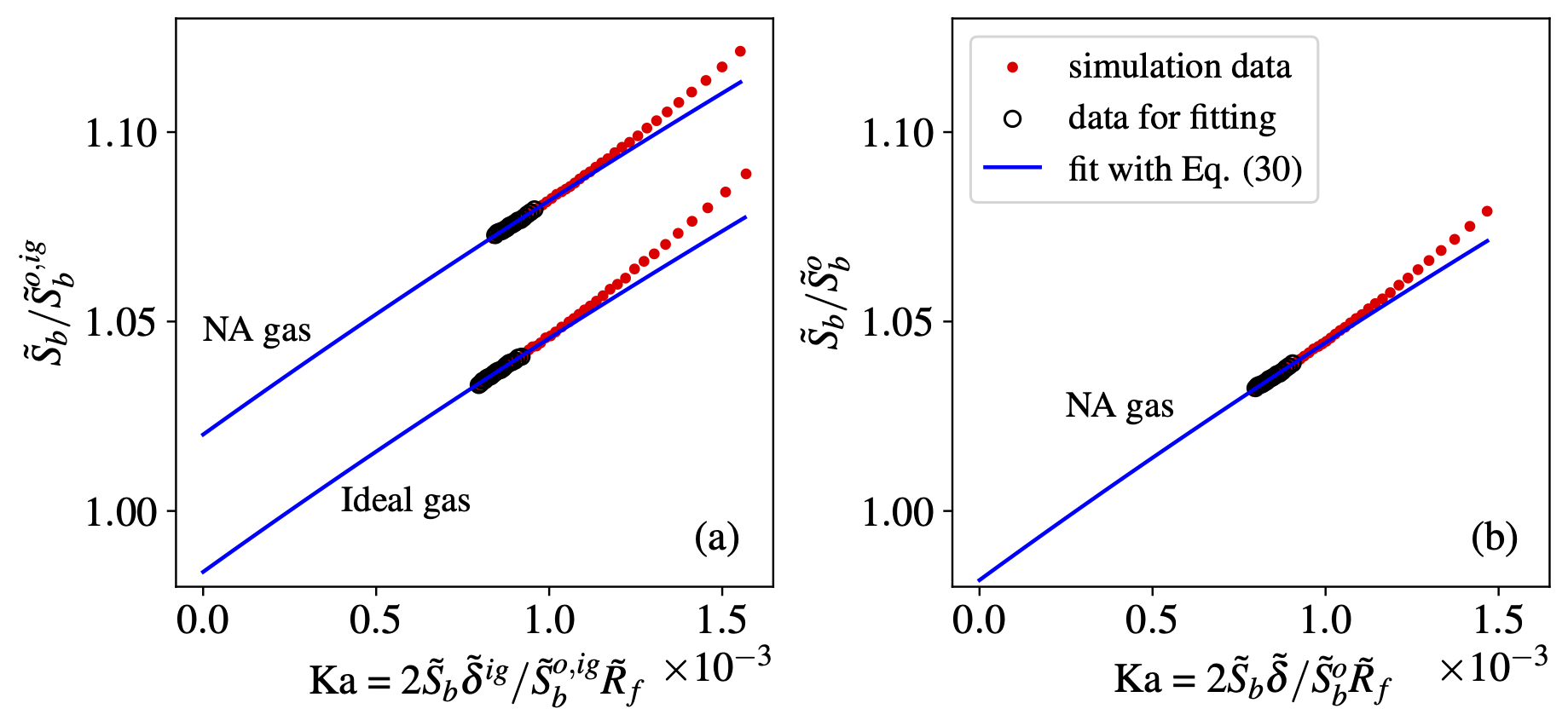
At high pressure, the real gas effects should be considered for the laminar flame speed, a fundamental combustion property for hydrogen flame. Relation between stretch rate and flame speed was derived via asymptotic analysis and Noble-Abel model.
\begin{equation} \label{eq:Evolution_S_R} \left(\frac{S}{1+b}\right)^{2}\ln\left(\frac{S}{1+b}\right)^{2} = -\frac{d}{dR}\left(\frac{S}{1+b}\right) + \frac{2}{R}\left(\frac{S}{1+b}\right) -\frac{d}{dR}\left(\frac{S}{1+b}\right) -l. \end{equation}
The Markstein length (number) was given as
\begin{equation} L_{b} = -\frac{\delta_{ad}I\beta}{2}= -\frac{\delta_{ad}\beta}{2}\int_{\varepsilon}^{1} \frac{1+b}{T^{0}+b}\left[\left(\frac{T^{0}-\varepsilon}{1-\varepsilon}\right)^{\mathrm{Le}-1}-1\right] {\rm d} T^{0}. \end{equation}
The extrapolation relation was validated with spherical flame simulation based on recently developed solver for real gas laminar flame.
[1] P.D. Ronney, and G.I. Sivashinsky, “A Theoretical Study of Propagation and Extinction of Nonsteady Spherical Flame Fronts,” SIAM J. Appl. Math. 49(4), 1029–1046 (1989).